|
Janjua Aneurysm Saddle
The patented Janjua Aneurysm
Saddle parent-vessel device involves first expanding a laterally-expandable
member, such as a balloon or resilient patch, within the parent vessel
over the neck of the aneurysm and then expanding a radially-expandable
member, such as a porous stent, in the parent vessel. It is not approved
by the FDA or available for patient use. Janjua
Aneurysm Saddle concept video.
The stent compresses the
laterally-expandable member against the aneurysm neck in order to seal
the aneurysm. This device can be especially useful for treating wide-neck
aneurysms without blocking nearby branching vessels. Figures 4 through
6 provide a three-sequence series of deployment views of one possible embodiment
of the Janjua Aneurysm Saddle.
Figure 4 shows a longitudinal
cross-sectional view (on the left) and a lateral cross-sectional view (on
the right) of the first stage of deployment of the Janjua Aneurysm Saddle
into the parent vessel of the aneurysm. The aneurysm is shown protruding
from the top of a parent blood vessel. Smaller blood vessels are
also shown branching off from the bottom of the parent vessel.
In this example, the device
includes an upper catheter and lower catheter. The upper catheter
delivers a laterally-expandable member such as an expandable, relatively-flat
balloon. In other examples, this upper catheter may deliver a relatively
flat mesh, net, lattice, membrane, layer of fabric, layer of shape memory
material, or patch of compressible material. The lower catheter delivers
a radially-expandable structural member, such as a stent. The lateral
cross-sectional view (on the right) more clearly shows how the flat balloon
has been inwardly-curved to fit into the catheter before its release and
lateral expansion. The lateral cross-sectional view also more clearly
shows the cross-sectional stent with an expansion-powering balloon in its
core.
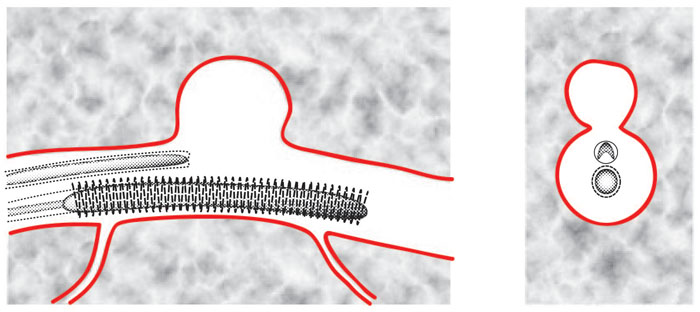
Figure 4: Janjua Aneurysm
Saddle: Insertion into Parent Vessel of Aneurysm
(longitudinal cross-sectional
view and lateral cross-sectional view)
Figure 5 shows the second
stage of deployment of the Janjua Aneurysm Saddle, wherein the upper balloon
has been pushed forward out of catheter and laterally expanded so that
it underlies the neck of aneurysm. This lateral expansion is more clearly
seen in the lateral cross-sectional view (on the right).
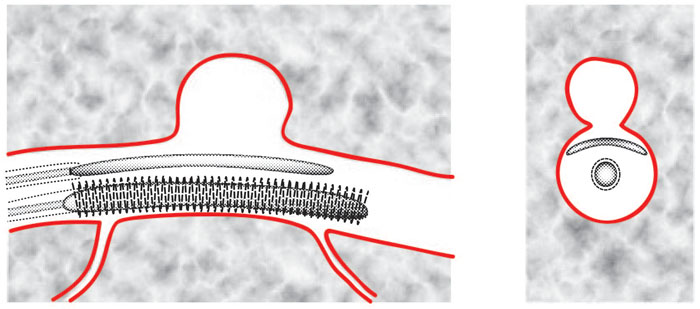
Figure 5: Janjua Aneurysm
Saddle: Deployment of Upper Balloon
(longitudinal cross-sectional
view and lateral cross-sectional view)
Figure 6 shows the third
stage of deployment of the Janjua Aneurysm Saddle wherein the stent has
been deployed from the lower catheter and both catheters have been withdrawn.
Expansion of the stent presses the upper balloon against the neck of aneurysm
to seal off the aneurysm. The formation of a "saddle" shape by the
upper balloon over the neck of the aneurysm is the origin of the descriptive
name for this device. The stent is sufficiently porous to allow
blood flow to branching vessels. The ability to selectively block
blood flow to the aneurysm, but not block blood flow to nearby branching
vessels, is an advantage of this invention over prior art involving stents
with uniform porosity.
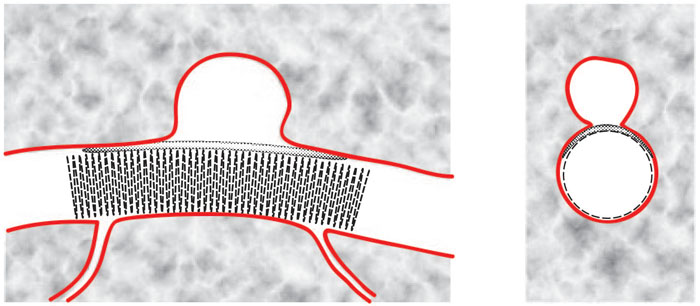
Figure 6: Janjua Aneurysm
Saddle: Final Deployment
(longitudinal cross-sectional
view and lateral cross-sectional view)
Expansion of the stent member
occurs after, or concurrently with, expansion of the upper balloon.
This provides more thorough coverage and sealing of the aneurysm neck than
is possible with devices and methods in the prior art wherein a stent is
expanded first and then embolic members are inserted through the wall of
the stent into the aneurysm. In such devices and methods in the prior
art, embolic members may be "jailed" in the aneurysm, which is good for
avoiding their prolapse into the parent vessel, but there can be gaps between
the inserted embolic members and the perimeter of the aneurysm neck, which
can lead to recanalization. These gaps and this recanalization can
be greatly reduced, or even avoided entirely, by the Janjua Aneurysm Saddle
device shown here. |